PROBLEM SET
1 Cell Cycle
1.1 Introduction
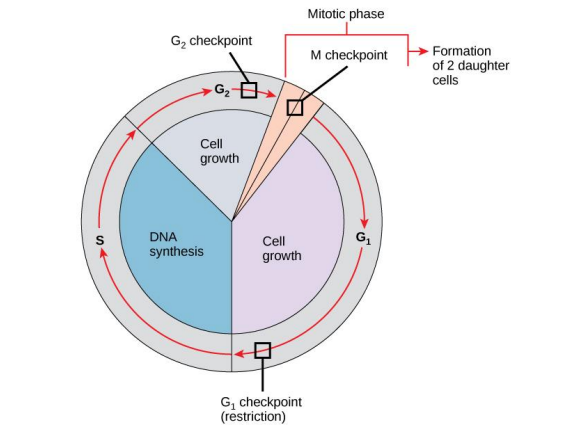
Cell cycle control is focused mainly on two events: the replication of genomic DNA and its
subsequent segregation between daughter cells, which occur during distinct cell cycle phases in
eukaryotic cells. The process of cell division is broken up into two stages: mitosis (M) and in-
terphase. Stages of mitosis include prophase, metaphase, anaphase, and telophase. Under the
microscope, interphase cells simply grow, but different techniques revealed that the interphase
includes G1, S and G2 phases.
Questions:
- Draw the cell cycle paradigm, clearly label M stage, interphase stages, G1, S, and G2
phases. If possible, specify the specific steps in mitosis - Clearly label the three checkpoints of cell cycle
2 Control of Cell Cycle: G1/S phase
2.1 Cell Cycle Entry – Positive Feedback Loop
Before S phase, in the pre-replicative G1 phase, there is a decision window during which cells
can commit to initiate DNA replication and enter the cell cycle or stay in G1 phase. Cell
cycle progression is driven by the accumulation of cyclin-dependent kinase (CDK) activity dur-
ing interphase and M phase. In cell cycle entry, the Start checkpoint integrates multiple in-
ternal and external signals into an all-or-none decision to enter the cell cycle. However, this
might not be the case. Here, we will use a series of experiments to revisit this idea and ques-
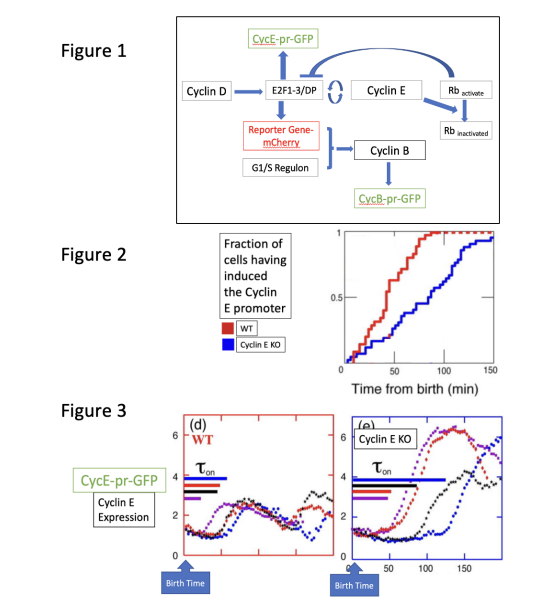
tion the model proposing a linear cascade of the activation. The model we will use is in a
transcription positive feedback of cyclin E in G1, which induces the near-simultaneous
expression of ~200 genes in G1/S regulon. Figure 1 proposes an initial model of signalling
transduction pathway.
As a reporter for Cyclin E transcription, we use unstable GFP driven by theCyclin E promoter
(CycE-pr-GFP). Figure 1 provides a general signal transduction pathway for reference. To
determine whether the positive feedback loop of G1-cyclins, using our GFP reporter gene con-
struct, we recreated this classical signal pathway in human cell lines. We have generated
modified cell strains containing Cyclin E knockout as our experimental cell lines. Birth time
was determined using a marker of Myo1-GFP myosin ring. In figure two, we plotted the populational cumulative distribution of CycE-pr-GFP induction, measuring the CycE-
pr-GFP-dependent positive feedback’s contribution to the early regulation of Cyclin E. In addi-
tion, we use single cell assay to plot the cell population. Each line with a different color
represents a cell line.
Note: Preliminary data have shown that Myo1-GFP markers have no influence on CycE-pr-GFP. The timing of Cyclin E promoter induction in individual cells is quantified computationally.
Question 1. Based on the first experiment (Figures 1&2), what can we observe? Is this in line
with the positive feedback loop that we expected to see?
Question 2. Based on the signal transduction pathway (Figure 1) and Figure 3, why Cyclin E KO cells express a more intense and prolonged CycE-pr-GFP signal? Is this in line with the positive feedback loop that we expected to see?
Question 3. Does the difference between Figure 2 and Figure 3 allow us to question the current linear model of activation cascade? Briefly explain.
2.2 Cell Cycle Entry – Negative Feedback Loop
We then wanted to determine if Cyclin-dependent positive feedback operated through the Retinoblas-
toma (RB) Tumor Suppressor, Rb, a transcriptional inhibitor of the G1/S regulon. RB is
known as a master regulator of the cell cycle. This transcriptional regulator exerts its function
in cell cycle control through its interaction with the E2F family of transcription factors and with
chromatin remodelers and modifiers that contribute to the repression of genes important for cell
cycle progression. RB binds to E2F family members at the promoters of genes important for S
phase progression and cell proliferation. The binding of RB to E2F proteins either blocks the
recruitment of transcriptional co-activators or recruits transcriptional co-repressors to these pro-
moters, thus repressing the expression of these genes and halting the G1/S cell cycle transition.
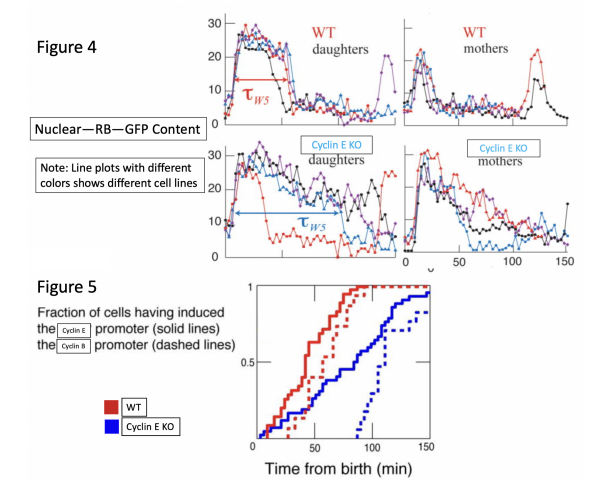
A quantitative assay for nuclear levels of RB-GFP has been performed, marking the nucleus with
H2B-mCherry and measuring the background noise ratio.
Question 4. Based on the line plot (Figure 4), what does a sudden increase of Nuclear-RB-GFP content represent? What does a decrease represent?
Question 5. Why, within each group, are there two graphs, daughters and mothers?
Question 6. Based on the four line plots, what can you say about Cyclin E and Rb interactions?
Question 7. One might speculate Cyclin D can be a kinase that serves as the regulatory component of Rb activation. Using Figure 1 as a model, propose what will happen to the ReporterGene-mCherry in WT cells and Cyclin D knockout (Tet-on).
Question 8. Let us revisit Figure 2. To further elucidate the signalling pathway of Cyclin E, we examined the Cyclin B promoter using CycB-pr-GFP (dashed lines). (Note: Preliminary data have shown that CycB-pr-GFP markers have no influence on CycE-pr-GFP.) Using Figures 1&5, briefly explain why Cyclin B is expressed later than Cyclin E.
Solution
Introduction
1,2: Answer may vary.
Control of Cell Cycle: G1/S phase
- Slowed accumulation of Cyclin E, faster accumulation in WT. Yes.
- Due to delayed onset of E2F mediated transcription. Yes.
- Open to multiple reasonings. Yes. The expression level varies in temporal and quantitative level. Current hypothesis proposes all-or-nothing activation may not be the case.
- Activation of a transcription factor. Deactivation of a transcription factor.
- Open to multiple reasonings. Mother cells divide into two daughter cells. Presenting two data sets could help researchers better understand epigenetic differences among cell lineages.
- Cyclin E can effectively reduce the fast deactivation/nuclear export of RB.
- Open to multiple reasonings. One possibility is premature death. Another possibility is Cyclin D KO will effectively reduce the accumulation of Cyclin B.
- Activation of Cyclin B needs G1/S regulon, which can be more downstream and more tightly regulated of the signaling pathway than Cyclin E.



